Causes of congenital stridor.
Causes of congenital stridor. |
Causes of congenital stridor.
Causas de estridor congénito.
Carlos Fernando Ríos Deidán1, Karen Sofía Flores Mena2, Edgar Vinicio Escalante Fiallos2, Tamara Michelle Acosta Castillo3, María Mercedes Narváez Black1.
INTRODUCTION. Airway abnormalities are rare but potentially fatal. Stridor is a respiratory noise with greater predominance in the inspiratory phase. OBJECTIVE. To evaluate the etiology of stridor, determine its comorbidities and mortality. MATERIALS AND METHODS. Retrospective cross-sectional study. Population of 110 and sample of 33 data from the Medical Records of neonatal or infant patients who presented stridor at the Carlos Andrade Marín Specialties Hospital of Quito-Ecuador, from january 2009 to december 2020. RESULTS. The 51,51% (17; 33) of cases were men. The age of the first consultation for stridor was within the first month in 18,00% (6; 33) and 40,00% (13; 33) at 3 months. The most frequent congenital laryngeal pathology was: laryngomalacia 81,82% (27; 33), followed by subglottic stenosis 9,09% (3; 33), bilateral chordal paralysis 6,06% (2; 33) and tracheal stenosis 3,03% (1; 33). The 51,51% (17; 33) presented comorbidities of causes: neurological, pulmonary and genetic among the main ones. Mortality was 18,20% (6; 33) related to the severity of comorbidities, except one secondary to tracheal stenosis. CONCLUSION. Laryngomalacia and subglottic stenosis were the predominant pathologies with congenital stridor. The comorbidities that occurred were neurological, pulmonary, genetic and caused mortality within 90 days after diagnosis.
Keywords: Respiratory Sounds; Laryngomalacia/congenital; Vocal Cords; Laryngostenosis; Congenital Abnormalities; Neonatology.
INTRODUCCIÓN. Las anomalías de la vía aérea son poco frecuentes, pero potencialmente mortales. El estridor es un ruido respiratorio con mayor predominio en la fase inspiratoria. OBJETIVO. Evaluar la etiología del estridor, determinar sus comorbilidades y la mortalidad. MATERIALES Y MÉTODOS. Estudio transversal retrospectivo. Población de 110 y muestra de 33 datos de Historias Clínicas de pacientes neonatos o lactantes que presentaron estridor en el Hospital de Especialidades Carlos Andrade Marín de Quito - Ecuador, de enero 2009 a diciembre 2020. RESULTADOS. El 51,51% (17; 33) de casos fueron hombres. La edad de la primera consulta por estridor fue dentro del primer mes en el 18,00% (6; 33) y del 40,00% (13; 33) a los 3 meses. La patología congénita laríngea más frecuente fue: laringomalacia 81,82% (27; 33), seguida de estenosis subglótica 9,09% (3; 33), parálisis cordal bilateral 6,06% (2; 33) y estenosis traqueal 3,03% (1; 33). El 51,51% (17; 33) presentaron comorbilidades de causas: neurológica, pulmonar y genética entre las principales. La mortalidad fue 18,20% (6; 33) relacionada con la severidad de las comorbilidades, excepto una secundaria a estenosis traqueal. CONCLUSIÓN. La laringomalacia y la estenosis subglótica fueron las patologías que predominaron con estridor congénito. Las comorbilidades que se presentaron fueron neurológica, pulmonar, genética y causaron mortalidad dentro de los 90 días posteriores al diagnóstico.
Palabras clave: Ruidos Respiratorios; Laringomalacia/congénito; Pliegues Vocales; Laringoestenosis; Anomalías Congénitas; Neonatología.
Airway abnormalities are rare, but life threatening1. They are identified by noisy breathing, cyanosis, apnea, thoracic retractions, difficulty in feeding and recurrent aspiration2. Delaying the diagnosis promotes lung damage that can lead to death2.
During embryological development, the larynx and vocal cords (VC) meet at the confluence of the digestive and respiratory tracts3. In the 12th week of gestation, the primitive laryngopharynx is compressed bilaterally with its lateral walls approaching the center of the lumen, which will eventually merge and give rise to the epithelial lamina, which after a reorganization stage will form the tracheoesophageal septum that would disintegrate to form the laryngotracheal tube3. Finally, a ventral (laryngeal) and dorsal (pharyngoepiglottic) recanalization event occurs.
The laryngeal cartilages and muscles complete their development before the separation of the vocal cords. Figure 1.
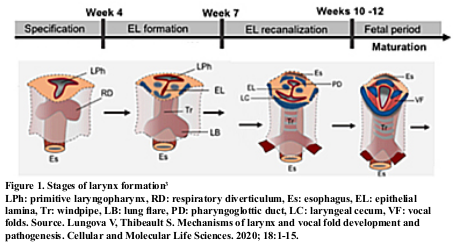
Failure of vocal cord recanalization results in laryngeal membranes or atresias producing polyhydramnios, whereas laryngeal clefts lead to chronic interstitial lung damage related to aspiration and gastroesophageal reflux3; therefore, this pathology alters the development of lung maturation.
The stridor is a respiratory noise, which occurs mainly during inspiration and results from the passage of a turbulent flow through an area of partial obstruction of the airway, which can be found at a supraglottic, glottic or subglottic level4, or due to extrinsic compression; with repercussion on breathing, swallowing and/or phonation.
It is important to differentiate it from abnormal respiratory sounds as described in table 1. If a biphasic stridor is heard (on inspiration and expiration) it suggests greater severity, the extent, both in diameter and length, of the obstruction determines the tone of the stridor and the intensity increases when the speed of the air flow is greater, such as during crying5. The SPECS algorithm facilitates a rapid and adequate clinical assessment of important signs of this pathology (Table 2), indicating the need for endoscopic evaluation5.
The main cause of stridor is the laryngomalacia and there are three theories6 that explain its etiopathogenesis: Neurological theory: neuromuscular incoordination resulting from sensory dysfunction caused by collapse of the supraglottic tissue; Anatomical theory: the larynx is in a higher position at birth and the laxity of the epiglottis coupled with a short aryepiglottic fold leads to supraglottic collapse during inspiration; Cartilaginous theory: the flexibility of the immature cartilage can worsen the 2 previous theories, giving rise to the collapse that produces the stridor and obstruction of the airway6.
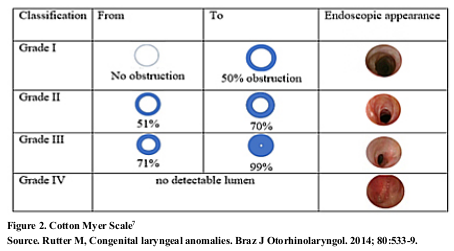
The subglottic stenosis is another congenital cause; it is evaluated with the Cotton Myer scale (Figure 2), it comes from a failure in the recanalization of the laryngeal lumen at week12; it is defined as a lumen 4,0 mm in diameter or less at the level of the cricoid in a full-term newborn7.
Membranes or pseudomembranes represent 5,0%8 of all laryngeal abnormalities. Most are glottic with subglottic extension and occupy mainly the anterior commissure. Treatment is directed according to severity in: clinical management, VC and subglottis surgery.
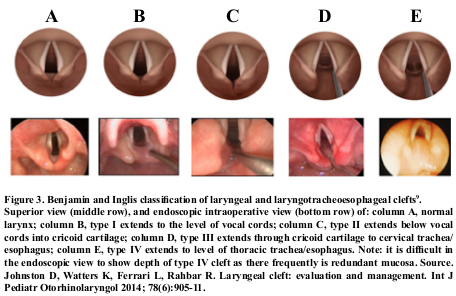
The laryngeal cleft represents 0,5 to 1,5%8 of all malformations; it is the incomplete separation between the esophagus and the airway from the beginning of the larynx, due to abnormal fusion of the posterior cricoid lamina and defective development of the tracheoesophageal septum. It is classified in 4 degrees (Figure 3)9. The clinical treatment is indicated in grade I (food thickening, swallowing rehabilitation and treatment of gastroesophageal reflux) which may eventually be associated with laryngoplasty injection and endoscopic repair. From grade II on, its resolution is purely surgical, preferably endoscopic10 and the open surgical technique is used mostly for grade III-IV (70,0% of cases)10.
Subglottic hemangiomas are rare, with a mortality of 50,0%6. More than half present as cutaneous hemangiomas synchronous to the subglottic lesion. Treatment varies between the use of: systemic or intralesional corticosteroid, atenolol11 or propranolol (3 mg/kg/day, between 1 to 15 months depending on the evolution)12 and finally surgical management with laser5, or combinations of all of them. Tracheostomy is indicated to preserve the airway when the management described above fails7.
Therefore, the objective was to determine its etiology, associated comorbidities and mortality in patients born with stridor at the Carlos Andrade Marín Specialties Hospital (HECAM) in Quito - Ecuador.
Retrospective cross-sectional study. Population of 110 data from Medical Records with International Classification of Diseases Codes (CIE) 10: Q311, Q312, Q313, Q314, Q315, Q318, Q319, and sample of 33, belonging to neonatal or infant patients who presented stridor, between January 2009 and December 2020 at the Carlos Andrade Marín Specialties Hospital in Quito-Ecuador. The inclusion criteria were stridor, newborn and infant; those who did not meet these criteria were excluded. Data tabulation and analysis were performed in Excel.
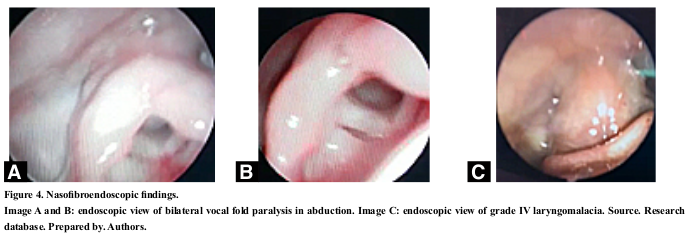
51,51% of the cases were men (17; 33). The age at the first consultation in the first month was 18,18% (6; 33), between the first 3 months 40,00% (13; 33), at 6 months 55,10% (18; 33) and 96,96% (32; 33) in the first year. The diagnosis was made within 15 days after the first appointment by flexible nasofiber endoscopy in 88,00% (29; 33) of the cases (Figure 4) and 12,12% (4; 33) by bronchoscopy. 65,50% (22; 33) of these studies were carried out in the hospital, the difference was referred to an external provider. The evaluation was completed in 4 patients with 3D airway tomography (subglottic and tracheal stenosis) to assess the extent of the stenosis.
From a demographic point of view; 87,87% (29; 33) of the patients came from the north-central area of the country with an altitude greater than 2 200 meters above sea level (m.a.s.l.) and the remaining 12,13% (4; 33) less than 800 m.a.s.l.
The most frequent congenital pathology that caused stridor was laryngomalacia (Table 3); it was present in 52,00% in males; with a mortality of 14,8%, related to the underlying comorbidity, followed by subglottic stenosis that corresponded to Cotton Myer III in all cases.
Vocal cord paralysis was abducted and bilateral, all male and associated with: corpus callosum agenesis, peritalamic leukomalacia, and cardiological abnormalities. At follow-up, one patient recovered cordal mobility at 8 months and the other died.
In 51,52% (17; 33) of the cases were found: genetic syndromes, neurological pathology and heart disease. Thirty one associated pathologies were described, and up to 3 diseases were identified in the same patient; as described in table 3.

Overall mortality was 18,20%, (6; 33) at 3 months of follow-up, all due to comorbidities, except for one case, related to underlying tracheal pathology.
The follow-up of the cases was an average of 3 years. Grade II-IV laryngomalacia and bilateral cord paralysis resolved during the first year of follow-up under expectant clinical management. The patient with grade IV laryngomalacia declined to grade II four months after diagnosis, but tracheostomy was performed due to his underlying myopathic pathology. The cases with subglottic stenosis presented severe respiratory distress requiring tracheostomy and subsequent referral to an external provider for definitive surgical resolution; being two decannulated.
The case of tracheal stenosis was managed first with tracheostomy and then dilations were performed with resection of the stenotic rim, unsuccessful; with death due to cardiopulmonary disease secondary to the third month of follow-up.
Respiratory distress is a common problem in newborns with a prevalence of 7,0%6, forty six percent die while breastfeeding; three-quarters during the first week of life. Stridor is one of the frequent presenting signs in newborns and the nasofibroscopic study is essential for its timely diagnosis. In the present study, there was a predominance in men in relation to the data reported by Contreras I, et al.4 with 55,6%.
Laryngomalacia was the most frequent pathology with 81,82%, in contrast published studies that presented up to 75,0%4,13. Cuestas G, et al.8 found a male predominance with a ratio of 2: 1.
96,0% of patients with laryngomalacia had spontaneous resolution one year, data related to the study by Rutter M7 who determined resolution between 12 to 18 months, Bedwell J and Zalzal G13 identified that between 10-15% of cases had symptoms severe and the surgery was performed between 3,8 and 5,5 months. The surgical criteria are apnea, cyanosis, pulmonary hypertension, cor-pulmonale, flat or decreasing growth curve and aspiration14. The procedures described were supraglottoplasty or epiglottopexy with cold or laser technique, within the framework of laryngeal microsurgery. Supraglottoplasty presented success rates that varied between 53-95%13, complications were less than 10,0%13. Failures were generally associated with incomplete resection of supraglottic tissue, genetic and neurological comorbidities, with a relative risk of failure of 7,1415; when this occurs, tracheostomy is the procedure to ensure airway13; as occurred in the study described.
A mortality of 14,8% was found, related to comorbidities, the literature showed rates lower than 2,00%13 in severe grades without comorbidities. A greater association between mortality and comorbidities was identified, a hypothesis that should be correlated in future studies.
The second cause of stridor was subglottic stenosis. In severe cases, if intubation fails, tracheostomy is the salvage procedure. For diagnosis, assessment of the level and thickness of the stenosis was performed: nasofibrolaryngoscopy and tomography with 3D reconstruction of the upper and lower airway; procedures performed in this study. Swallowing evaluation was indicated in case of high suspicion of aspiration. Tracheostomy was performed as initial treatment in all patients with grade III stenosis and definitive treatment was performed in an external hospital; no mortality. The decision for surgical resolution was made after a thorough analysis of the patient’s condition, comorbidities and stenosis characteristics. Grade I or II stenosis has a greater probability of success than dilations with an endoscopic balloon16,17, 80,0%18 in grade II and 50,0% in grade III. Open reconstructions such as laryngotracheoplasties or resection with end-to-end anastomosis are indicated, when dilations fail, grade III, IV or multilevel stenosis16 and the tracheostomy maintains the patency of the airway when these techniques described are not successful6,7.
Congenital tracheal stenosis is rare and potentially fatal, with an incidence of 1 in 64 500 live births19, the severity is variable according to: the extent and degree of stenosis, concomitant pulmonary and bronchial involvement. Recanalization of the lower airway is the objective of management; the risk factors for surgical failure are: generalized hypoplasia and cardiovascular comorbidities15. With an operative mortality of 16-20%20. In this study, a case was reported, in which the treatment was not successful.
Bilateral fold paralysis is the inability to move with the consequent obstruction and generation of stridor. This study represented the third cause with 6,25% of the cases, in the literature it was described between 15-20%2,5,21, all were men, in contrast to 63,0% described by Scatolini M, et al.22. Regarding etiology; 55,0% are idiopathic, 17,0% traumatic21 and secondary to neurological malformations between 11-43%22,23, being Arnold Chiari disease the most frequent21; in this study were: agenesis of the corpus callosum and peritalamic leukomalacia, also described by other authors23. The spontaneous recovery rate was 46-64% according to reported data22,21 during the first year, correlated with the result of this study; and up to 10,0%22 after 5 years. Indications for emergent surgical management were: failure to thrive and apnea crisis with cyanosis. Techniques varied between: endoscopic (cordectomy24, uni or bilateral, arytenoidectomy25, cricoid splint26) and open approaches (laryngotracheoplasty with posterior costal graft22). When airway is not acceptable in the long term, tracheostomy is the treatment of choice.
The most frequent pathologies presented with stridor were: laryngomalacia subglottic stenosis and vocal cord paralysis. Approximately half of the cases presented neurological, pulmonary and genetic comorbidities, which are associated with mortality within the first 3 months after diagnosis.
Emphasize early diagnosis based on a comprehensive evaluation of each patient to initiate timely treatment. Provide a child nasofibroscopy for the Unit Medic to avoid making referrals.
CIE: International Classification of Diseases codes; HECAM: Carlos Andrade Marín Specialties Hospital; m.a.s.l: meters above sea level; VC: Vocal cords.
CR, KF, EE, TA, MN: Conception and design of the work, Analysis and interpretation of data, Drafting of the manuscript, Critical revision of the manuscript, Approval of the final version of the manuscript, and Accountability.
Carlos Fernando Ríos Deidán. Doctor in Medicine and Surgery, Universidad Central del Ecuador. Specialist in Otorhinolaryngology, Universidad San Francisco de Quito. Specialist in Otorhinolaryngology, Otorhinolaryngology Unit, Carlos Andrade Marín Specialties Hospital. Professor, Postgraduate in Otorhinolaryngology, School of Medical Sciences, Universidad Central del Ecuador. Quito-Ecuador. ORCID: https://orcid.org/0000-0001-6120-0004
Karen Sofía Flores Mena. Surgeon, Pontificia Universidad Católica del Ecuador. Quito-Ecuador. Specialist in Otorhinolaryngology, Universidad Central del Ecuador. Specialist in Otorhinolaryngology, Otorhinolaryngology Unit, Carlos Andrade Marín Specialties Hospital. Quito-Ecuador. ORCID:https://orcid.org/0000-0002-2789-2067
Edgar Vinicio Escalante Fiallos. Medical Doctor, Universidad Central del Ecuador. General Physician in Hospital Functions, General Teaching Coordination, Carlos Andrade Marín Specialties Hospital. Quito-Ecuador. ORCID: https://orcid.org/0000-0001-6668-3471
Tamara Michelle Acosta Castillo. Medical Doctor, Universidad Central del Ecuador. Postgraduate in Otorhinolaryngology, Eugenio Espejo Specialties Hospital. Quito-Ecuador. ORCID: https://orcid.org/0000-0002-5400-4336
María Mercedes Narváez Black. Doctor
in Medicine and Surgery, Universidad
Central del Ecuador. Specialist in Otorhinolaryngology,
Universidad Técnica
Particular de Loja. Master in Health
Services Management, Escuela Politécnica
Nacional. Otorhinolaryngologist,
Otorhinolaryngology Unit, Carlos Andrade
Marín Specialties Hospital. Professor,
Postgraduate in Otorhinolaryngology,
School of Medical Sciences,
Universidad Central del Ecuador. Quito-
Ecuador. ORCID: https://orcid.org/0000-0003-5170-0703
Free bibliographic resources were used, in addition to the Medical Records authorized by the patient. The information collected is available upon request to the main author.
The study was approved by peers and by the Human Research Ethics Committee-CEISH/HCAM.
The publication was approved by the Editorial Policy Committee of the CAMbios Scientific Medical Journal of the HECAM in Act 002 dated May 20, 2021.
The authors’ own resources were used.
The authors reported having no personal, financial, intellectual, economic or corporate conflicts of interest.
To the teamwork achieved for the satisfactory completion of this article.